- Home
- Product Properties
- FDA-Bond 2 Food & Drug Administration Medical Grade Epoxy Adhesive, Low Viscosity RT Cure
- Home
- Product Properties
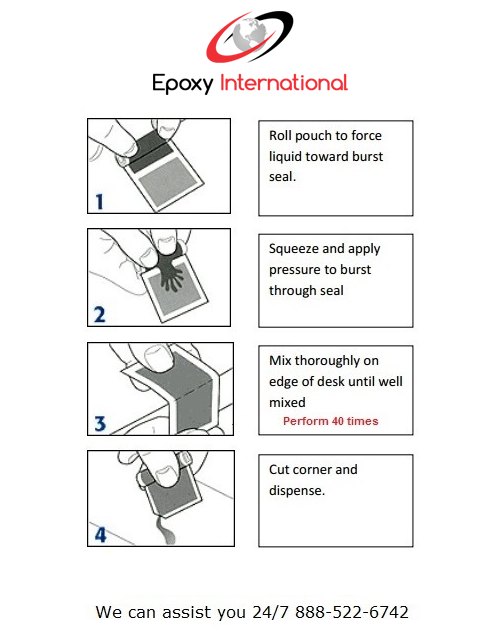
- Easy Dispense
- FDA-Bond 2 Food & Drug Administration Medical Grade Epoxy Adhesive, Low Viscosity RT Cure
- Home
- FDA Grade
- FDA-Bond 2 Food & Drug Administration Medical Grade Epoxy Adhesive, Low Viscosity RT Cure
- Home
- Product Properties
- Medical Grade
- FDA-Bond 2 Food & Drug Administration Medical Grade Epoxy Adhesive, Low Viscosity RT Cure
- Home
- Product Properties
- FDA Grade
- FDA-Bond 2 Food & Drug Administration Medical Grade Epoxy Adhesive, Low Viscosity RT Cure
FDA-Bond 2 Food & Drug Administration Medical Grade Epoxy Adhesive, Low Viscosity RT Cure
$4.99
Product Description
FOOD AND DRUG APPLICATION EPOXY ADHESIVE
Product Reviews
Write Review
-
Great product

Posted by Unknown on Feb 18th 2018
While repairing an old Melmac plastic bowl from the 1960's wouldn't be worth the effort to some, this product enabled pinpoint accuracy and ease in application. When used as suggested it cured to bind the three sections of the bowl together for a permanent and barely visible repair.
 Loading... Please wait...
Loading... Please wait...